Purple beans are really quite special. These are my favorite type of “green” bean because they’re so easy to pick. The pods are brightly colored and stand out among the leaves. They look great next to all the emeralds, chartreuse, and hunter greens in the garden.
Please note that if you buy something after reading this article or clicking on one of my links, I may get paid a small fee.
Purple beans are a fun and colorful variety of bean to grow and eat. However, many people are surprised when their vibrant purple beans turn green during cooking. What causes this chameleon-like change? Let’s explore the science behind why purple beans turn green.
What Are Purple Beans?
Purple beans, also called royal burgundy or purple snap beans, are a cultivar of the common green bean plant (Phaseolus vulgaris) They are identical to green beans except for their distinctive deep purple pod color This eye-catching color comes from natural plant pigments called anthocyanins. Anthocyanins provide the blue, red, and purple hues found in many fruits, vegetables, and flowers.
Raw Purple Beans Are Purple
When purple beans are raw, the high concentration of anthocyanins masks the green chlorophyll in the pods. This allows the vibrant purple anthocyanins to dominate, creating a rich royal purple color. The cells of raw purple bean pods also contain highly acidic sap, which intensifies the anthocyanin pigments.
So in the raw state the anthocyanins’ purple color is boldly on display in purple beans. But what happens when they are cooked?
Why Cooked Purple Beans Turn Green
When purple beans are cooked two major changes occur that cause them to turn from purple to green
-
Heat degrades the anthocyanins.
-
Cell walls rupture, diluting the acidic sap.
Heat Decomposes Anthocyanins
Anthocyanin pigments are sensitive to heat. Exposure to high temperatures during cooking breaks down and destroys the anthocyanin compounds. With the purple-providing pigments degraded, the purple color fades.
Cell Rupture Neutralizes Acidity
Cooking also ruptures the cell structure of the bean pods. This liberates the acidic, anthocyanin-rich sap inside the cells. The released cell contents mix with cooking water, raising the pH and making the environment less acidic. More neutral pH conditions cause the purple hue to disappear.
As the two factors of anthocyanin degradation and pH change occur, the green chlorophyll in the beans becomes visible. Chlorophyll was present all along but overpowered by the vibrant purple. The chlorophyll emerges as the dominant pigment when anthocyanins are destroyed and acidity reduced from cooking, causing the color shift to green.
Preserving Purple Hue
Cooking methods that minimize heat exposure can help preserve more purple color. Try steaming, microwaving or stir-frying purple beans briefly. Acidic ingredients like lemon juice or vinegar can also stabilize the anthocyanins. However, the purple-to-green transformation will occur to some extent with any cooking. Enjoy purple beans for their chameleon nature and nutrition!

How and why anthocyanins change color in the garden
You may have noticed that purple beans’ color changes a little from season to season or doesn’t look the same as other plants you’ve seen if you’ve ever grown them yourself. The purple stripes may be more or less noticeable, and the pods may be very dark purple one year and lighter the next.
Anthocyanins change color naturally because they are sensitive to the pH level of the “juice” (cell sap) inside plant cells. The acidity of the cell sap is dependent on both genetic and environmental factors.
Anthocyanins tend to turn red in acidic soil, blue in neutral soil, and yellow in alkaline soil.
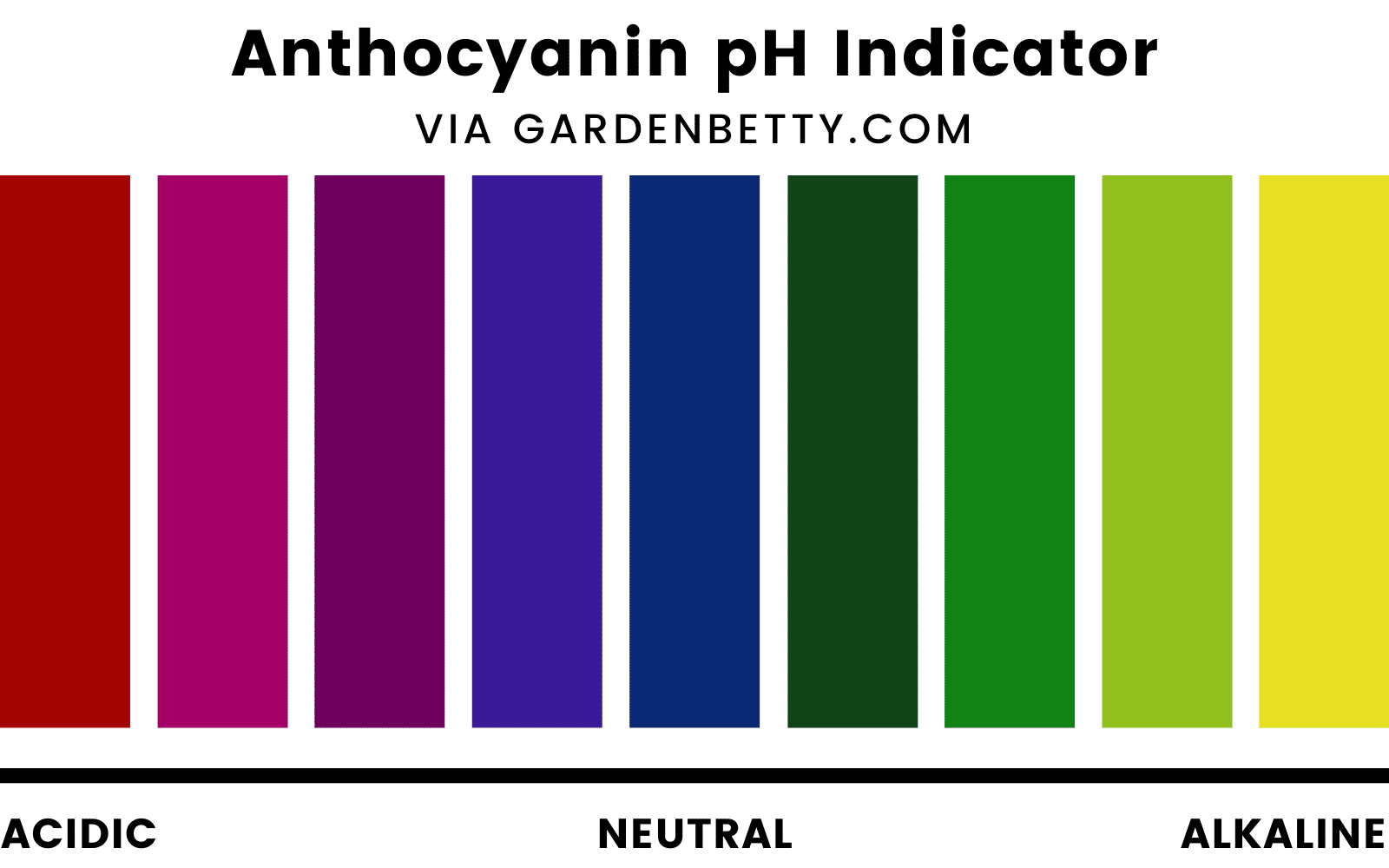
This is why “red” cabbage may appear more purple, and “purple” cauliflower may appear more magenta.
It’s also why hydrangeas are known for changing colors; the petals react very differently to different soil types. In fact, gardeners can easily change the color of the flowers by making the soil more or less acidic.
Think of these anthocyanin-rich plants as a litmus test of sorts. (In fact, red cabbage makes a good indicator in pH experiments at home.)

Where do purple beans get their color?
Anthocyanins are plant pigments that give string beans and snap beans (like Royal Burgundy and Dragon Tongue) their deep purple color. Yardlong beans (like Chinese Red Noodle) and hyacinth beans (like Ruby Moon) also have this color.
(But note that anthocyanins are only responsible for certain reds. You can see red colors in some plants, like beets and chard. These colors come from betalains, which are only found in Caryophyllales plants. Other red pigments, like those in red peppers and red tomatoes, come from carotenoids. ).
There are anthocyanins in every part of a plant. They can be found in the stems, leaves, flowers, fruits, and roots (but not all at once).
With purple beans, the pigments primarily show up in the flowers, which gradually give way to purple-tinged pods.

Tip Of The Day: Purple Beans
FAQ
Are purple green beans OK to eat?
Why do purple peppers turn green when you cook them?
What’s the difference between green beans and purple beans?
Why do anthocyanins turn green?
